Download CBSE Science Sample Papers Class X (10th) 2008
SAMPLE PAPER – 2008
Class - X
SUBJECT - SCIENCE (Theory)
(set-14)
Q 1)What do you mean by alkalis? Give example of an alkali. 1 Marks
Q 2)At old age the lens of people becomes milky and cloudy. Name the condition. What harm can be caused by this condition? 1 Marks
Q 3)How many periods and groups are there in the modern periodic table? 1 Marks
Q 4)See the reaction below and answer which of the substance is getting reduced and which of the substance is getting oxidised?
Q 5)How many joules are in 1 watt-hour? 1 Marks
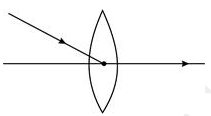
Q 6)Redraw the diagram to show the refracted ray.
Q 7)Differentiate between alternating current and direct current.
2 Marks
Q 8 )Draw the electron dot structure for ethane. 2 Marks
Q 9)Give reactions with water to show that magnesium is less reactive than sodium? 2 Marks
Q 10)You are given three resistors of equal resistance R. How will you combine them to have (i) maximum resistance (ii) minimum resistance? Also find the ratio of maximum resistance to minimum resistance.
2 Marks
Q 11)Give reasons for:
(i) Decomposition reactions are called endothermic reactions.
(ii) Antioxidants are added to foods containing fats and oil.
(iii) Sodium and potassium are kept in kerosene oil. 3 Marks
Q 12)(a) Label the following marked parts in the given diagram.
(b) What type of image is formed by the eye lens on the retina? 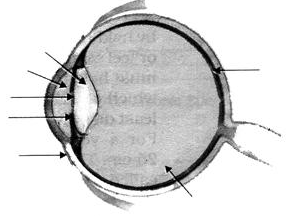 3 Marks
3 Marks
Q 13)An object 6 cm tall is placed at a distance of 24 cm in front of a convex lens of focal length 16 cm. Find the position, nature and size of the image. 3 Marks
Q 14)(a) What are the reasons for a large number of compounds of carbon? (b) A compound X burns with a clean flame whereas a compound Y burns with a sooty flame. Which of the two is a saturated carbon compound? Which of the two will show an addition reaction? 3 Marks
Q 15)(a) State one limitation of each, Newland’s law of octaves and Mendeleev’s Periodic table.
(b) An element A with atomic number 12 forms SO42- with the formula ASO4. Identify the element and its place in the periodic table. What will be the formula of its chloride?5Marks
Download Attached PDF
- General Sanskrit - 1
(300 downloads)